Additive Masterbatch; the best way to improve physical and mechanical properties of polymer products with recycled raw materials (Part 1)

Additive Masterbatch; the best way to improve physical and mechanical properties of polymer products with recycled raw materials (Part 1)
As consumption of polymer products grows, the need for the production of polymer raw materials increases, accordingly. We know that most of the polymers are made in petrochemical companies, which are considered non-renewable resources. On the other hand, environmental pollution caused by leaving polymer products in nature is increasing. An efficient solution to respond the need for polymer raw materials as well as non-destruction of the environment is the recycling of polymer products. In the recycling of polymers, polymer wastes, after classification and separation and washing, are reheated and formed in several stages. This method allows us to manage wastes and energy very much and also protect natural resources [1].
Limitations in the recycling of polymers
In theory, all polymers can be recycled, but in practice, there are obstacles that can make this process difficult. This process is not always environmentally, economically and technically cost-effective; Some of the main reasons are discussed below:
- In most cases, polymer products are mixture of different types of polymers and they have several layers that are difficult to separate and cost a lot.
- Polymers are often contaminated by food or other substances and the resin to be produced is not very clean for reuse.
- Millions of dollars must be spent to build and operate facilities used in recycling, and it is beneficial if a large amount of materials are recycled daily. Very small amounts make the recycling process not economical and beneficial due to low efficiency and high costs.
Despite these definitions, the recycling process can be done and useful products can be produced. For example, the milk containers that we consume, plastic bottles, shopping bags, etc. can be turned into usable products by recycling.
Recyclable polymers
- Polyethylene terephthalate PET:
Currently, the most commonly recycled polymer is PET. However, for some countries, achieving an appropriate recycling rate is still a challenge. For example, Europe, India, and South Korea have reached rates above 50%, but China and the USA still recycle very small amounts of used PET.
The latest global statistics published in 2011 tell us that approximately 7.5 million tons of PET were collected. But what do these materials turn into? Most of them become items that can be used in the fashion industry. Among the products we can produce are woolen clothes, backpacks and even carpets. The interesting tip is that some recycled plastic bottles can be turned into a beautiful t-shirt. In the recycling process, PET containers are turned into small pieces and then spun like yarn and can be used as a raw material in the production of clothing and textile industries. Also, PET bottles can be turned back into PET bottles! In fact, they are polymers that can be recycled over and over in the same form.
 Figure 1. A shampoo bottle made from recycled PET [2]
Figure 1. A shampoo bottle made from recycled PET [2]
Another use of recycled PET is house construction. For example, a house in Canada was built by melting 600,000 plastic bottles and molding them.
 Figure 2. House made with recycled PET [3]
Figure 2. House made with recycled PET [3]
2. HDPE High density polyethylene:
HDPE is known as one of the easiest polymers to recycle and is accepted in most recycling centers. In America, the recycling rate for this material is about 30%. The purpose of HDPE recycling is to use for non-food containers; Such as detergents, household cleaners, etc. Also, painted HDPE is used in the production of pipes. In some cases, recycled HDPE can be turned into other products such as benches and other durable plastic products.
- LDPE Low density polyethylene:
LDPE polyethylene is used to produce plastic bags that are given to people by grocery stores and retailers. Technically, LDPE can be recycled, but as mentioned before, recycling should be economically cost-effective, but products made with LDPE are light and cheap, and recycling them may not be economically justified. Also, plastic bags get tangled in recycling machines, which make the recycling process difficult. Despite these problems, recycled LDPE can be used in packaging films.
- Polypropylene PP:
Polypropylene, abbreviated as PP, is one of the materials used in the packaging industry, and only 3 to 5 percent of it is recycled in the United States, and most of it ends up in landfills. It takes about 20 to 30 years to degrade and decompose. We may ask ourselves that if this material is recycled, why do we throw it away? Unfortunately, it is not economically viable to recycle it; It is very difficult and expensive to recycle, and it is also very difficult to get rid of its smell. Recycled PP is seen in gray or black color, which is not suitable for packaging at all. Anyway, recycled PP is used to make car parts, park benches, speed bumps, etc.
- PS polystyrene:
Polystyrene recycling is very difficult but not impossible; As we know, polystyrene is used for the food packaging industry and contains a lot of contamination from food, which makes it very difficult to recycle.
In the following table, a summary of the applications of recycled polymers is categorized:
Table 1. Applications of recycled polymers [4]
| Polymer | Application |
| PET | Detergent bottles, packaging films, carpet fibers |
| HDPE | Detergent bottles, mobile parts, agricultural pipes, toys |
| LDPE | Plastic tubes, food packaging, bottles |
| PP | Car parts, park benches, speed bumps |
| PS | Disposable spoons and forks |
According to published statistics, the production of polymer waste in the world is increasing, which is depicted in Figure 3, the amount of production of polymer waste from 1950 to 2015.
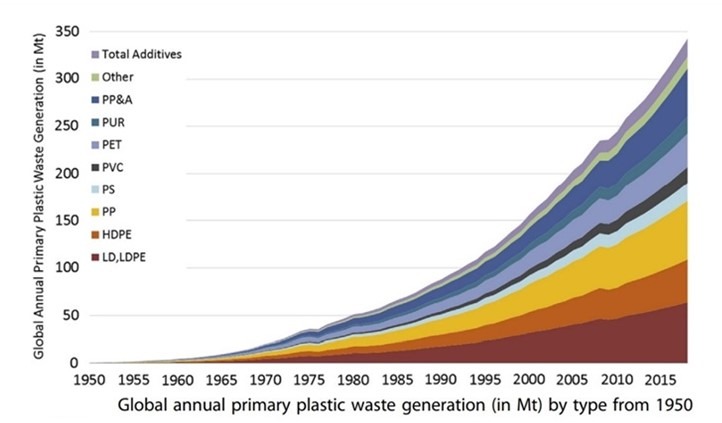 Figure 3. Amount of polymer waste production in the world from 1950 to 2015 [5]
Figure 3. Amount of polymer waste production in the world from 1950 to 2015 [5]
Before 1980, Recycling of polymer waste was negligible, meaning that 100% of polymer waste was thrown away. After that, part of the waste was burned and recycled since 1990; This rate is increasing at a rate of 0.7% per year, so it is estimated that 55% of the world’s polymer waste is landfilled, 25% is incinerated, and 20% is recycled. By 2050, this figure is expected to reach 50% incineration, 44% recycling and only 6% discarding. In the graphs below, the said process can be seen (Figure 4).
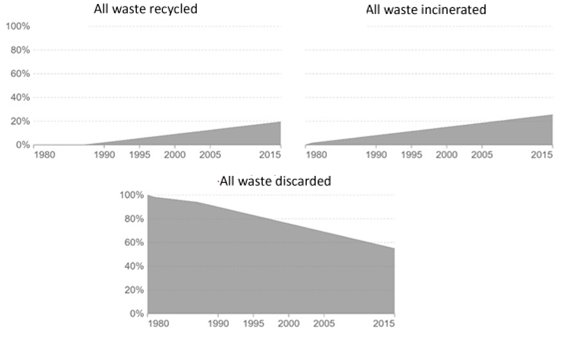 Figure 4. The rate of recycling, incineration and discarding of polymer waste [6]
Figure 4. The rate of recycling, incineration and discarding of polymer waste [6]
The question may arise, what is the contribution of each polymer in the production and recycling process? As seen in Figure 5, the highest percentage of polymer production is dedicated to polyethylene polymers. After that, polypropylene and polyethylene terephthalate are in the next positions.
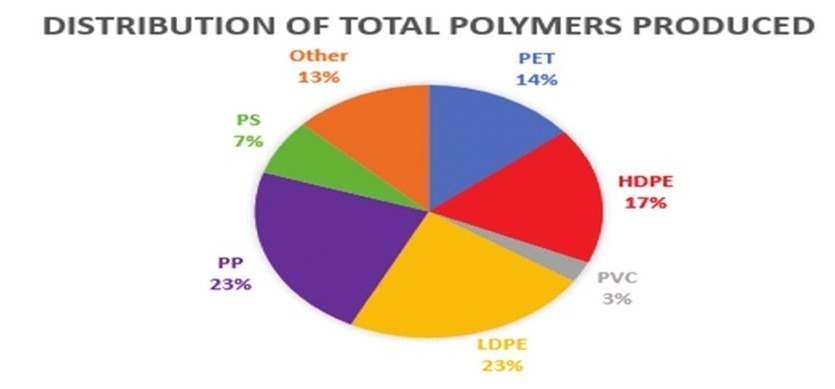 Figure 5. Percentage of production of polymers in the world.
Figure 5. Percentage of production of polymers in the world.
According to the collected statistics shown in Figure 6, PET has the largest share in the recycling of polymers, followed by polyethylene.
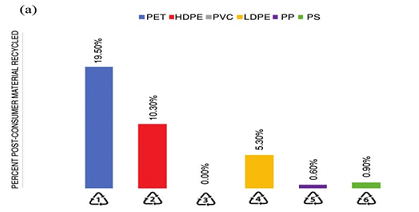 Figure 6. Percentage recovery of different polymers.
Figure 6. Percentage recovery of different polymers.
Investigating the mechanical properties of recycled polyolefins
Although recycling polymers is beneficial for the environment and economy, the main goal is to get the same efficiency of raw polymers (untouched virgin) in recycled polymers. The best type of recycling to have maximum energy efficiency and minimum environmental consequences is mechanical recycling. However, there are a number of differences between virgin and recycled polymers. Due to the structural changes and the presence of impurities in the polymer and its rendering, it is difficult to achieve a quality recycling. Whether the recycled polymer is suitable for obtaining new applications or not, is measured by mechanical tests (such as tensile test, impact strength), physical tests (such as hydraulic stability, surface roughness) and operational tests (extrusion, molding) under standard conditions. When the above tests are performed, most recycled polymers do not meet the requirements required for various applications, unless we use additives that improve their properties. However, to understand how much recycled polymers are far from the desired properties; Let’s look at some examples:
- PP: The molecular mass of polypropylene is significantly reduced by reprocessing and recycling, affecting the mechanical properties. For example, a battery case made from recycled materials took just 16 days to break down; But the battery produced with intact polypropylene lasted 38 days. The elongation at break of the sample with intact polymer was 680%, but only 20% for the recycled sample.
- PE: Polyethylene tend to cross-link with reprocessing and aging. The most important problems that polyethylene have in the outdoor space are cracking and breaking. For example, a trash can made of virgin HDPE had a strength of 404 KJ/m2 in the tensile impact test, while the recycled sample had a strength of 291 KJ/m2.
- PS: When polystyrenes are exposed to heat, their molecular mass decreases and color changes are also observed.
Therefore, to increase the physical, mechanical and appearance properties of recycled polymers, it is necessary to add additives that improve these properties. Among these, the focus in this article is on the widely used additive in polyolefin as the most recycled polymer.
Additives to improve the properties of recycled polyolefins
Usually, recycled polymers do not have good properties without adding additives. The addition of additives should improve the life and appearance of recycled polymers. For reformulation with different specifications, a full range of additives is available for virgin polymers. We can mention examples such as compatibilizers, impact modifiers, stabilizers, etc. In addition to affecting the polymer matrix, additives also affect the overall properties of the composite. Researchers have focused more on the stability and compatibility of recycled polymers in relation to additives. In Table 2, the types of additives used in recycled polyolefin polymers are categorized along with their performance.
Table 2. Types of additives and their performance for polyolefins [6].
| Function | Additive type |
| Dilution of recycled material, increase of physical and mechanical properties | raw polymer (primary) |
| Increasing the compatibility of the polymer mixture, along with increasing the physical and mechanical properties | Compatibilizers |
| Increased processability, more melt flow | Lubricants, waxes |
| Increasing processability, long-term thermal stability | stabilizers (antioxidants) |
| Increased optical stability | Stabilizers (amines, ultraviolet stabilizers) |
| Increasing thermal stability in the presence of metal impurities | Metal deactivators |
| Appearance | Illuminators and pigments |
| Increasing physical and mechanical properties | reactive molecules |
| Improvement of mechanical properties | Fillers, fibers |
| Improvement of mechanical properties, compatibilize | Coupling factors |
| odor reducer | Silicates (such as zeolite) |
| Adjust melt flow, change mechanical properties | Radical generators (such as CRPP) |
Reference
- https://insights.globalspec.com/article/14792/how-to-recycle-polymer waste#:~:text=Polymer%20recycling%20is%20a%20material,series%20of%20def ned%20sub%2Dprocesses.
- https://www.plasticsforchange.org/blog/which-plastic-can-be-recycled
- https://www.cbc.ca/news/canada/nova-scotia/plastic-bottle-home-nova-scotia-1.5188749
- https://www.mdpi.com/2313-4321/2/4/24#B64-recycling-02-00024
- Geyer, R. (2020). Production, use, and fate of synthetic polymers. Plastic Waste and Recycling, 13-32. https://doi.org/10.1016/B978-0-12-817880-5.00002-5
- Geyer, R., Jambeck, J. R., & Law, K. L. (2017). Production, use, and fate of all plastics ever made. Science Advances, 3(7), e1700782.



