Polycarbonates (PC) are condensation polymers having desirable properties such as high clarity, thermal stability, high heat distortion temperature, and in spite of their hardness, they are flexible instead of being brittle against impact. Having such properties placed them in engineering polymer groups. Polycarbonates are easily formed and different grades of them are obtained by processes such as extrusion, injection, and blow molding. So far, the most consumed polycarbonates, which are used in more than 90% of commercial usage, are produced from the reaction between bisphenol A and a dysfunctional, proton-accepting species such as diphenyl carbonate or phosgene. The common chemical structure of this kind of polycarbonate is shown in figure1 [1].
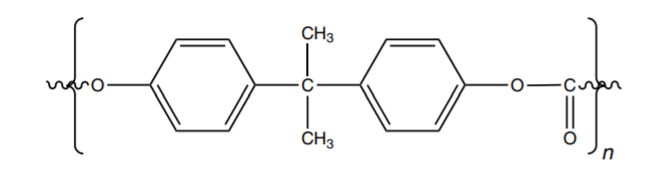
Figure 1: Chemical Structure of Polycarbonate Based On Bisphenol A. [1]
Bisphenol A, also called 2,2 Bis(4-hydroxyphenyl) propane as its official chemical name, is a dysfunctional monomer with two reactive hydroxyl groups (fig 2b) which polymerized with dicarbonyl monomers, such as phosgene or diphenyl carbonate (fig 2a). During the polymerization process, the hydroxyl group of bisphenol A deprotonate in a base condition, and then the oxygen atoms on the bisphenol A residue form ester bonds with dicarbonyl compounds (fig3). The polymerization process will be over when a monohydric phenol reacts with the end of the growing chain[1].
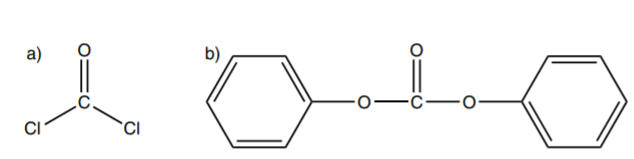
Fig.2: A) Phosgene B) Bisphenol A Monomer [1]
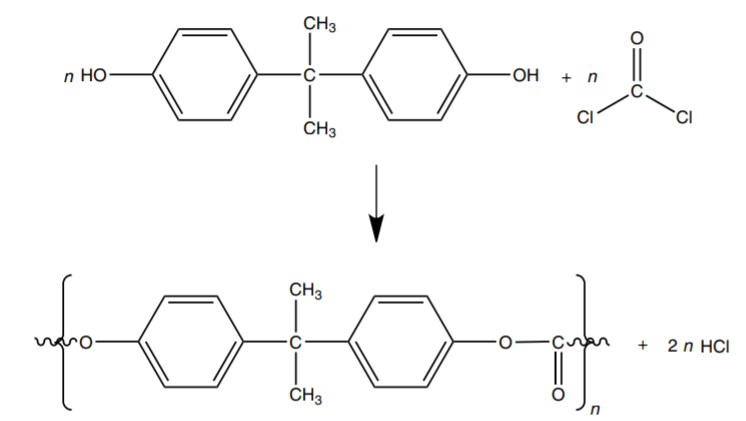
Fig 3: Polycarbonate Polymerization Based On Phosgene and Bisphenol A. [1]
Polycarbonates used in common manufacturing processes are not crystalline. Evidence from the local orientation of chains at the molecular level of polycarbonates suggests that repeating unites can be reassembled onto chains in a structure similar to letter Z. But these large-scale monomeric units do not associate with each other to form a regular crystalline material; and only under specific conditions, such as slow cooling of the polymer, small crystalline domains can be created. Since these conditions are not met in commercial processes, it is easy to say that generally, all the polycarbonates used in industry have an amorphous structure.
Polycarbonates can be manufactured by interfacial polymerization or through a melt esterification process in both batch or continuous conditions. The properties of polycarbonates can differ by different polymerization methods. For example, polycarbonates manufactured via interfacial polymerization are more stable in high temperatures and are less stiff than those produced via melt esterification. So while choosing a grade of polycarbonate resin for a particular application, it is crucial to know the method by which the polymer is produced.
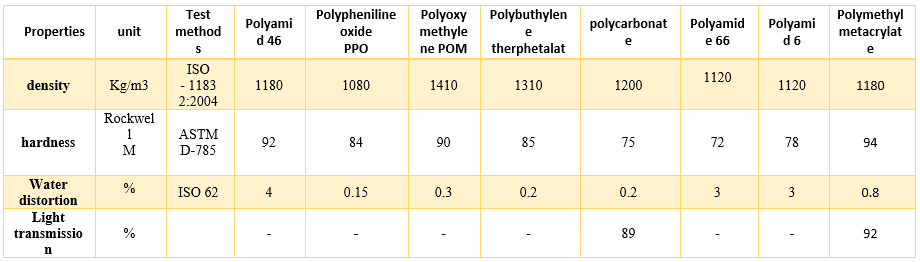
Typically, polycarbonates at a wide range of temperatures display high impact resistance, good thermal stability, excellent clarity and high modulus. For these reasons, they are used in many applications with challenging performance requirements, such as fire fighter’s helmets, power tools, and appliance housings, face shields, and automotive and aircraft panels; and they have received special attention compared to other unmodified engineering plastics (table 1). Negative aspects of polycarbonates are photo-degradation during exposure to ultraviolet and gamma radiation, low chemical resistance, and susceptibility to crazing especially when exposed to solvents, mechanical stresses, or high-temperature conditions [4].
Table 1: Comparison on Properties of Some Engineering Thermoplastics. [4]
The high modulus of polycarbonates even under high-temperature conditions is the result of the high glass transition temperature(Tg) of the polymer (141-150 °c). Under this temperature, the polymer is rigid and displays little distortion under load. By increasing glass transition temperature, the modulus can be improved in high temperatures, which can be achieved by a sensible selection of bisphenol. For increasing glass transition temperature, substituents can be added to phenol groups. In this way, by adding only one functional group to the phenol ring in the bisphenol, the Tg decreases. But by adding the second functional group to the ring, the segmental bonds are reduced and the Tg increases. Modification of bisphenol structure can also affect the stiffness of the final polymer. For example, the structure shown in figure 4 connects the two phenol rings to each other. This inflexible molecular structure creates a rigid polycarbonate backbone resulting in a stiffer polymer. So generally it can be concluded that the stiffness of the polymer increases by increasing the rigidity of a bisphenol section[1].
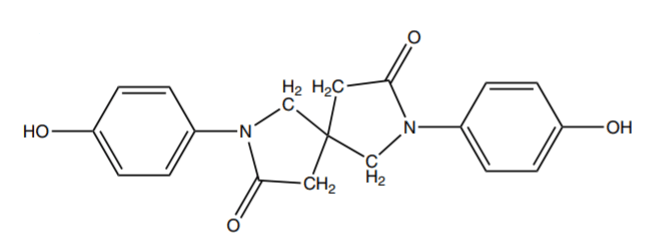
Fig.4: Chemical Structure of Spirodilactam Bisphenol.
The high clarity of polycarbonate is the result of its amorphous structure. There are no crystalline/amorphous interfaces that can scatter light which leads to opacity. Polycarbonate’s refractive index differs a little from glass, so it makes Polycarbonate a perfect substitute for glass. For example, drawers used in refrigerators are made of polycarbonate whose transparency, lightweight, and structural integrity mimic the appearance of glass similar to polycarbonate.
Polycarbonate has a unique birefringence behavior due to the orientation of small-scale polymer chains in injection molding components. It is seen as a rainbow effect in which the spectrum of colors is seen at certain angles. Injection-molded materials cause this phenomenon due to having highly ordered anisotropic skin.
Polycarbonates are resistant to alcohols, ordinary soaps, some oils, and gases, and dilute acids. But they are not resistant to dilute and strong bases, chlorinated solvents, organic ketones, and cyclic ethers (table 2). They can also crack parts if exposed to fatty acids as well as alcohol at high pressures [2].
Table 2: Chemical Resistance of Some Engineering Thermoplastics. [2]
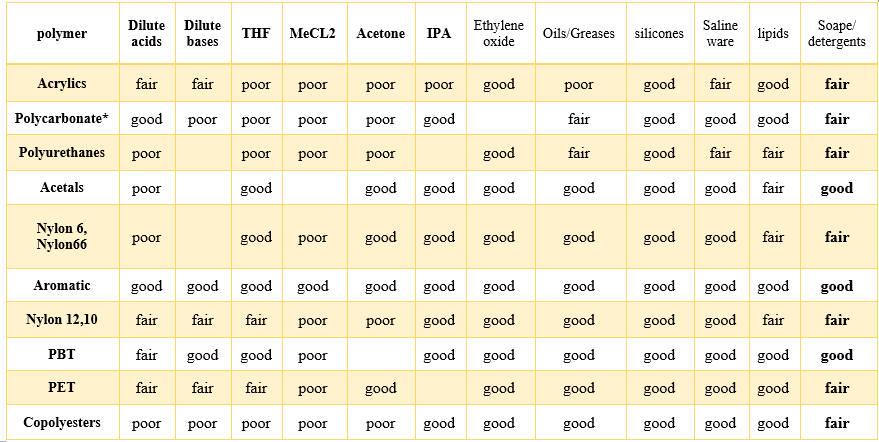
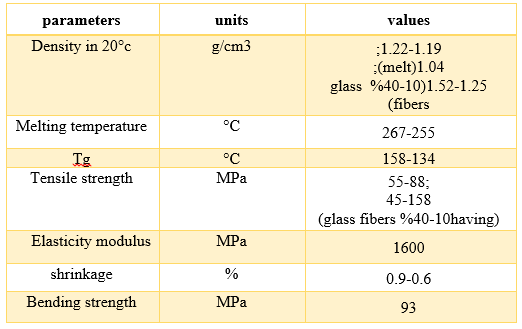
Table 3: Some of the Physical and Mechanical Properties of PCs. [3]
Applications of Polycarbonate:
Polycarbonates can be used in manufacturing CD/DVD, glasses, contact lenses, bulletproof windows, medical applications such as surgical instruments- drug delivery systems- hemodialysis membranes- blood vessels- blood filters, LED screens, car headlight housing. They are also used in direct contact with foods and beverages due to their heat resistance, crush resistance, and ability to match with the health regulations. Food containers made of polycarbonate are reusable, help to keep freshness, protect food from contamination, and can be easily used in refrigerators or microwaves.
Table 4: Samples of Polycarbonate Grades in Khuzestan Petrochemical Company. [5]
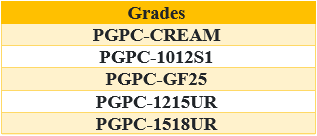
Currently, the main production of polycarbonate in Iran belongs to the Khuzestan petrochemical company. This company is the main supplier of raw materials used in industries mentioned above by producing PCs with different properties. Examples of different grades produced by this company are mentioned in the table below [5].
Author: Hanieh Tavakoli